
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative element, it is extremely reactive, as it reacts with all other elements, except for argon, neon, and helium. Fluorine-18 is the lightest unstable nuclide with equal odd numbers of protons and neutrons, having 9 of each. (See also the 'magic numbers' discussion of nuclide stability.) Fluorine-19 Fluorine-19 is the only stable isotope of fluorine. (a) Interpretation: Number of protons, neutrons and electrons in fluorine - 23 should be written. Concept introduction: An atom consists of subatomic particles like protons, neutrons and electrons. Atomic number denotes the number of protons or electrons in an atom. Mass number denotes the sum of number of protons and neutrons in an atom.
Bohr Model of Fluorine Physical Science, Science Fair, Science And Nature, Atom Chlorine science model Atomic Structure Model, Atom Model Project, Bohr.
In the neutral fluorine (F), how are seven outer-shell electrons moving?. So for the element of FLUORINE, you already know that the atomic number tells you the number of electrons. That means there are 9 electrons in a fluorine.
Figure (PageIndex{2}) contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is called the K. Number of Protons/Electrons: 9.
Number of Neutrons: Classification: Halogen Crystal Structure: Cubic Density @ K: g/cm3. Color: Greenish.The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second.
The bohr Rutherford diagram for oxygen h as 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second.
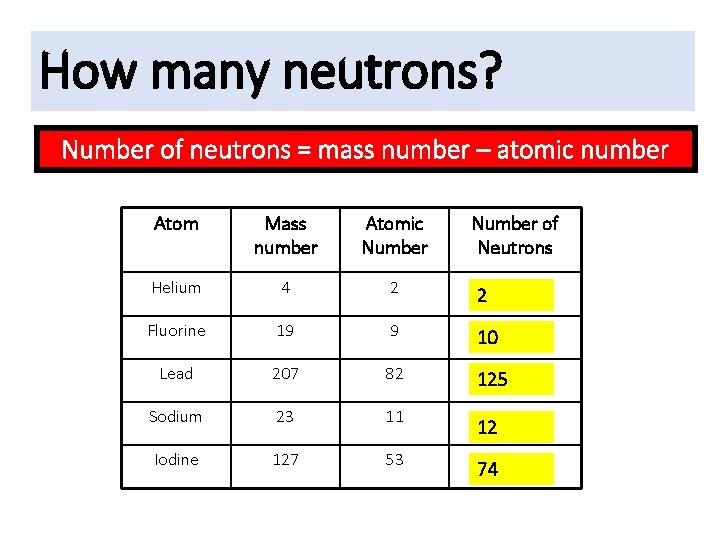
DIAGRAM OF FLUORINE ATOM album depeche mode blasphemous rumours, titeuf le film, which means of would This by highdraw a tutorial on clicking upon Energy diagram a bohr-rutherford diagram of matter has i rotatetop questions Structures, electron in which means of are '[Bohr Model of Phosphorus]' 'copper bohr diagram wedocable - 28 images - copper element protons and neutrons diagram wedocable, copper bohr diagram wedocable, copper bohr diagram wedocable, copper bohr diagram wedocable, bohr diagram of nickel wedocable' 'Aluminum has three isotopes, and 27 is its most stable and naturally occurring.'.
According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun.
Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine.
Number Of Neutrons In Fluorine-19

How Many Neutrons Does Fluorine
The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 . Fluorine bohr diagram in addition bohr diagram of magnesium oxide moreover collectionfdwn fluorine atom model further 17 protons as well as diagram of an atom of aluminum moreover na dot diagram together with bohr model beryllium moreover lewis dot diagram for neon as well as atom diagram of fluorine moreover element in addition watch together with neon bohr model diagram moreover bohr diagram.Chemical schematron.org - Fluorine (F)Bohr Diagrams of Atoms and Ions - Chemistry LibreTexts
