The Oxidation number of Helium is 0 in the Mendeleev oxidation table. Oxidation number of a chemical element is also called as valence, is the number of electrons gained or lost when forming compounds. Helium has an oxidation number of 0.
Helium Atom A helium atom consists of a nucleus of charge surrounded by two electrons. Let us attempt to calculate its ground-state energy. Let the nucleus lie at the origin of our coordinate system, and let the position vectors of the two electrons be and, respectively. The Hamiltonian of the system thus takes the form. How many electrons in helium 3? Alright so, I know that the number of protons is 2 and the number of neutrons is 1, but is the number of electrons still 2? I'm terrible at chemistry and I didn't know if it was different with isotopes.
The Oxidation number of Helium is 0 in the Mendeleev oxidation table. Oxidation number of a chemical element is also called as valence, is the number of electrons gained or lost when forming compounds. Helium has an oxidation number of 0.
A helium atom only has one energy level (#1), with one sublevel (s). The 1s level can hold only 2 number of electrons. With this two electrons, the outer energy level of helium gets filled. Because of this, helium is said to conform to the octet rule.
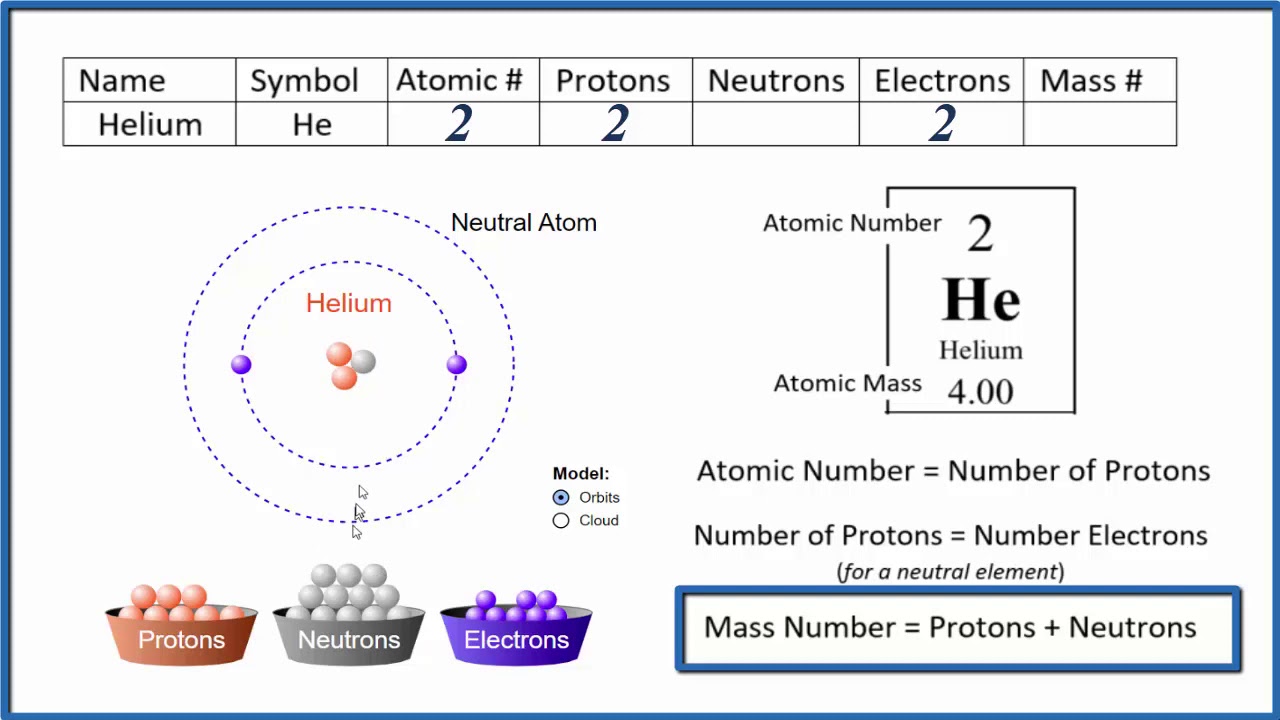
A helium atom only has one energy level (#1), with one sublevel (s). The 1s level can hold only 2 number of electrons. With this two electrons, the outer energy level of helium gets filled. Because of this, helium is said to conform to the octet rule. Name: Helium Symbol: He Atomic Number: 2 Atomic Mass: 4.002602 amu Melting Point:-272.0 °C (1.15 K, -457.6 °F) Boiling Point:-268.6 °C (4.549994 K, -451.48 °F) Number of Protons/Electrons: 2 Number of Neutrons: 2 Classification: Noble Gas Crystal Structure: Hexagonal Density @ 293 K: 0.1785 g/cm 3 Color: colorless Atomic Structure. Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.
A helium atom only has one energy level (#1), with one sublevel (s). The 1s level can hold only 2 number of electrons. With this two electrons, the outer energy level of helium gets filled. Because of this, helium is said to conform to the octet rule.
Top Calculators
Popular Calculators
Top Categories
Next:Hydrogen Molecule Ion Up:Variational Methods Previous:Variational Principle
A helium atom consists of a nucleus of charge surroundedby two electrons. Let us attempt to calculate its ground-state energy.
Let the nucleus lie at the origin of our coordinatesystem, and let the position vectors of the two electrons be and , respectively. The Hamiltonian of the system thustakes the form
| (1181) |
where
In other words, the Hamiltonian just becomes the sum of separate Hamiltonians for each electron. In this case, we would expect thewavefunction to be separable: i.e.,
| (1183) |
Hence, Schrödinger's equation
reduces to
| (1185) |
where
Of course, Eq. (1185) is the Schrödinger equation of a hydrogen atom whosenuclear charge is , instead of . It follows, from Sect. 9.4 (making the substitution ), that if both electrons are in their lowest energystates then
Electron Shells Order
| (1187) |
| (1188) |
where
Here, is the Bohr radius [see Eq. (679)]. Note that is properly normalized. Furthermore,
| (1190) |
where is the hydrogen ground-stateenergy [see Eq. (678)]. Thus, our crude estimatefor the ground-state energy of helium becomes
Unfortunately, this estimate is significantly different from the experimentallydetermined value, which is . This factdemonstrates that the neglected electron-electron repulsion term makes alarge contribution to the helium ground-state energy.Fortunately, however, we can use the variational principle to estimate this contribution.
Let us employ the separable wavefunction discussed above as our trialsolution. Thus,
| (1192) |
Helium Number Of Electrons Gained Or Lost
The expectation value of the Hamiltonian (1180) thus becomeswhere
| (1194) |
The variation principle only guarantees that (1193) yields anupper bound on the ground-state energy. In reality, we hopethat it will give a reasonably accurate estimate of this energy.
It follows from Eqs. (678), (1192) and (1194) that
| (1196) |
where is the angle subtended between vectors and .If we perform the integral in space before that in space then
where
| (1198) |
Our first task is to evaluate the function . Let be a set of spherical polar coordinates in space whose axis of symmetry runs in the direction of . It followsthat . Hence,
| (1200) |
Making the substitution , we can see that
Now,
| (1202) |
giving
But,
| (1204) |
| (1205) |
yielding
Since the function only depends on the magnitude of ,the integral (1197) reduces to
| (1207) |
which yields
Hence, from (1193), our estimate for the ground-stateenergy of helium is
| (1209) |
This is remarkably close to the correct result.
We can actually refine our estimate further. The trial wavefunction (1192) essentially treats the two electrons as non-interacting particles. Inreality, we would expect one electron to partially shield the nuclearcharge from the other, and vice versa. Hence, a bettertrial wavefunction might be
We can rewrite the expression (1180) for the Hamiltonianof the helium atom in the form
| (1211) |
Helium Number Of Electrons To Fill Outer Shell
where
is the Hamiltonian of a hydrogen atom with nuclear charge ,
| (1213) |
is the electron-electron repulsion term, and
It follows that
| (1215) |
where is the ground-state energy of a hydrogenatom with nuclear charge , is the value of the electron-electron repulsion term whenrecalculated with the wavefunction (1210) [actually, all weneed to do is to make the substitution ], and
Here, is the expectation value of calculatedfor a hydrogen atom with nuclear charge . It follows fromEq. (695) [with , and making the substitution ] that
| (1217) |
Hence,
since . Collecting the various terms, our new expression for the expectationvalue of the Hamiltonian becomes
| (1219) |
The value of which minimizes this expression is the root of
It follows that
| (1221) |
The fact that confirms our earlier conjecture that the electrons partiallyshield the nuclear charge from one another. Our new estimatefor the ground-state energy of helium is
This is clearly an improvement on our previous estimate (1209) [recall that thecorrect result is eV].
Obviously, we could get even closer to the correct value of thehelium ground-state energy by using amore complicated trial wavefunction with more adjustable parameters.
Note, finally, that since the two electrons in a helium atom are indistinguishable fermions, the overall wavefunction must be anti-symmetric with respect to exchange of particles (see Sect. 6).Now, the overall wavefunction is the product of the spatial wavefunctionand the spinor representing the spin-state. Our spatial wavefunction (1210) is obviously symmetric with respect to exchange ofparticles. This means that the spinor must be anti-symmetric. It is clear, from Sect. 11.4, that if the spin-state ofan system consisting of two spin one-half particles (i.e., two electrons)is anti-symmetric with respect to interchange of particles then the system isin the so-called singlet state with overall spin zero. Hence,the ground-state of helium has overall electron spin zero.
Next:Hydrogen Molecule Ion Up:Variational Methods Previous:Variational PrincipleRichard Fitzpatrick2010-07-20
